Advancing a pipeline of first-in-class therapies in indications where microcirculation could be transformative
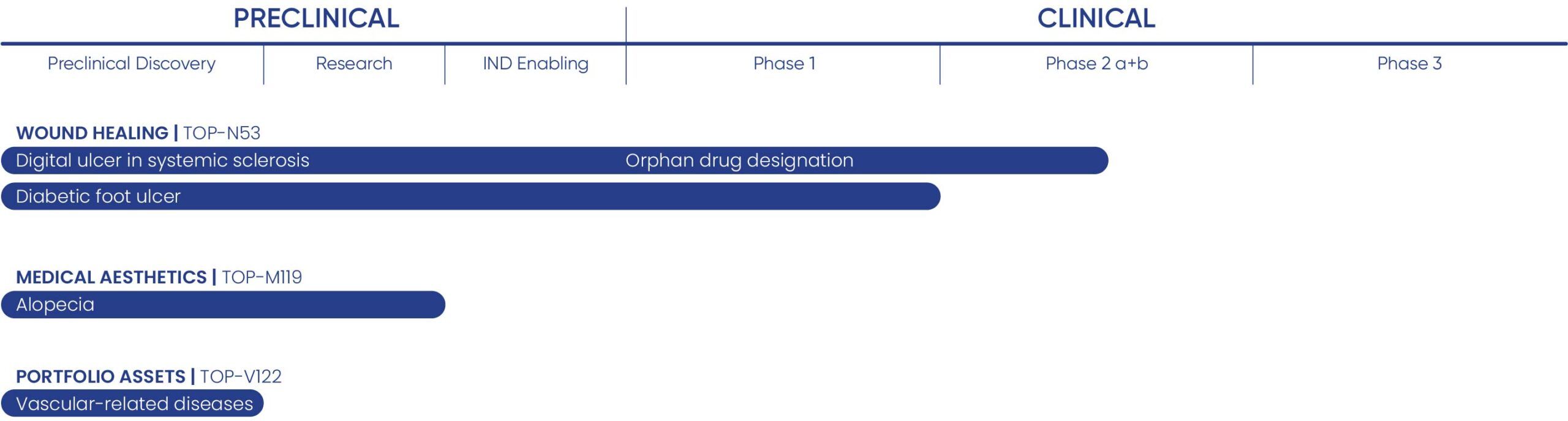
Topadur has a diversified pipeline of three first-in-class lead candidates targeting vascular-related conditions with high unmet medical need. Our portfolio of small-molecule drugs spans preclinical to Phase 2 clinical development, providing multiple opportunities for success and a steady flow of future product candidates.
Disease areas


Wound healing | Top-N53
Systemic sclerosis
Systemic sclerosis is a rare autoimmune disease affecting approximately 1.47 million people around the world. Causing skin tightening and impaired blood flow, it commonly is associated with digital ulcers – painful, chronic wounds typically impacting a patient’s fingers. For this debilitating condition there is currently no effective approved treatment and no late-stage topical therapy in development.
Diabetic foot ulcers
Patients suffering from diabetes often experience impaired microcirculation and nerve damage (diabetic neuropathy), leading to diabetic foot ulcers. Approximately 18.6 million people worldwide are affected each year. These ulcers can escalate into severe infections and even amputations – with a lower limb amputation occurring every 20 seconds globally. Current solutions and late-stage developments are not yet targeting the underlying microcirculation dysfunction.
TOP-N53 is a topical therapy currently in Phase 2a development for digital ulcers in systemic sclerosis (NCT06954597) and Phase 2 ready for the treatment of diabetic foot ulcers.
In a completed Phase 1 clinical trial, our lead compound has demonstrated improved local microcirculation and an excellent safety profile. In preclinical models, TOP-N53 has shown strong efficacy and no adverse effects at exposure levels well above the anticipated therapeutic dose in humans. These results point to a strong safety margin and high therapeutic potential.
TOP-N53 holds orphan drug designation from the American Food and Drug Administration (FDA) and European Medicines Agency (EMA) for digital ulcers in systemic sclerosis, underscoring its promise in this rare condition.

Medical aesthetics | TOP-M119
TOP-M119 is a topical candidate advancing toward clinical development for the treatment of androgenetic alopecia (hair loss) and other dermatological conditions associated with aging. It could emerge as the first non-hormonal, metabolism-based candidate with disease-modifying potential, and one of the first dual-pathway therapies designed to locally stimulate microcirculation and tissue regeneration.
In preclinical studies, TOP-M119 demonstrated superior efficacy, faster onset, and improved local tolerability compared to standard-of-care treatments. IND / CTA preparations for the start of clinical development are currently underway.
The program for the topical formulation is supported by a co-development and licensing agreement with Oshen Holdings SA for the Chinese market.
In addition, Topadur is developing intradermal sustained release formulations for use in medical cosmetics targeting alopecia and skin aging.
Research Portfolio | TOP-V122
Beyond these lead indications, Topadur supports additional in vivo proof-of-concept programs exploring conditions where improved microcirculation could be transformative – fueling a focused yet expandable innovation pipeline.
TOP-V122 demonstrated preclinical efficacy in models of colorectal cancer – an area of high unmet medical need. Its powerful angiogenic and regenerative activity also positions it as a compelling candidate for additional indications such as lung fibrosis and pulmonary arterial hypertension (PAH). In lung disease, TOP-V122 may offer disease-modifying potential – particularly in high-risk and refractory patients, including those awaiting lung transplants. In vivo studies are ongoing to expand the compound’s proof-of-concept.
»We are continuously evolving as we strive to develop groundbreaking therapies for rare and aging diseases that urgently need innovative solutions.«